Healthcare shipping solutions
From primary care to critical supplies, we’re ready to deliver.
From primary care to critical supplies, we’re ready to deliver.
Empowering patient care with innovative healthcare shipping
When you’re shipping medical products or pharmaceuticals, healthcare expertise isn’t just a competitive advantage—it’s critical. You need healthcare shipping services that seamlessly support integrity, quality, precision, and control.
Supporting timely results for immune health
It’s more important than ever for individuals to know their current immune health status. Watch FedEx deliver irreplaceable samples that give patients and physicians essential data to make decisions.
Urgent and everyday shipping services
Urgent healthcare delivery
When time is of the essence, we’ll get your urgent healthcare shipments there fast.
For envelopes, parcels, and packages weighing 150 lbs. or less:
- Get quick door-to-door delivery with FedEx SameDay® services—whether it’s blood samples or a pacemaker.
- Choose from three FedEx® overnight healthcare shipping services for convenient next-day delivery.
For shipments weighing 150 lbs. or more:
- Get FedEx SameDay U.S. healthcare delivery within hours, depending on flight availability.
- Opt for FedEx First Overnight® Freight and your shipment will arrive the next morning.
- Decide whether you want your time-sensitive shipment to arrive in 1, 2, or 3 business days.
Everyday healthcare shipments
Even when it’s not a rush, you still need secure and cost-effective shipping.
For envelopes, parcels, and packages weighing 150 lbs. or less:
- FedEx Ground® is an affordable way to ship to businesses typically within 1–5 business days. It’s faster to more locations than UPS Ground®. View service maps for estimated transit times.
- For Lab Returns, it’s important to bring your sample to a location or drop box the same day you collect it and before the last pickup for FedEx expedited shipping options. View locations and instructions here.
For shipments weighing 150 lbs. or more:
- Use LTL freight shipping to get delivery typically in 1–6 business days.
- Get delivery through the front door of a business or home via FedEx Freight Direct. It’s an ideal option for patients who need heavy medical equipment carried into a bedroom.
Shipping medical equipment, devices, and supplies
Whether you’re shipping a dialysis machine, dental retainers, or disposable gowns, we’ll safely deliver your medical products. With flexible transit times, costs, and service offerings, you can balance schedules and budgets. Here are some of the benefits of shipping your medical equipment, devices, or supplies with FedEx:
- Delivery to hospitals, homes, and medical facilities—even specific rooms
- Tracking and visibility for your most valuable shipments
- Custom packaging to protect delicate shipments
- Expertise in the cross-class regulatory landscape
- Decades of delivery expertise for medical equipment, devices, and supplies
- Add-on services for enhanced peace of mind
Delivery to hospitals, homes, and medical facilities—even specific rooms
Tracking and visibility for your most valuable shipments
Custom packaging to protect delicate shipments
Expertise in the cross-class regulatory landscape
Decades of delivery expertise for medical equipment, devices, and supplies
Add-on services for enhanced peace of mind
Shipping pharmaceuticals and biologics
If you’re shipping pharmaceuticals or biologics, we’ll provide exceptional care of raw materials or finished products. Get end-to-end precision with FedEx packaging, storage,* transparent data, and quality assurance.
No matter what you’re shipping, you’ll have more control over your inventory’s journey—and beyond. From warehousing to advisory guidance, take advantage of our supply chain and logistics services.
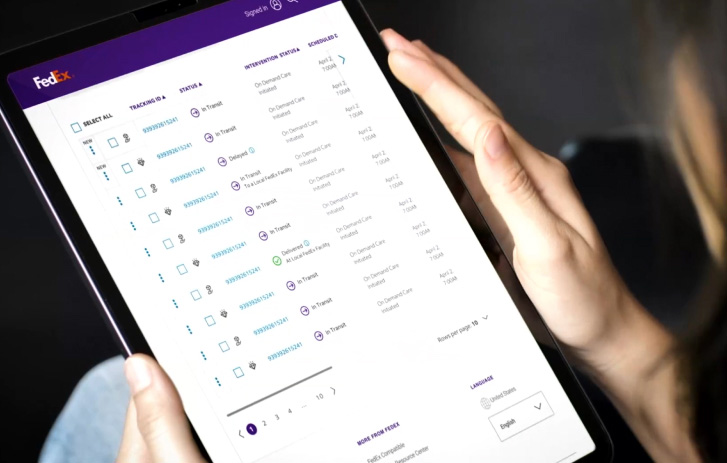
Shipment monitoring and intervention
With some shipments, you need heightened visibility, security, or quality assurance. We offer flexible healthcare delivery solutions that work for your budget and schedule.
Make proactive decisions with predictive insights
Clear shipment visibility puts you in the driver’s seat. The FedEx Surround monitoring and intervention suite gives you near real-time data. So you can prioritize shipments, request intervention, and mitigate risk.
Stay in constant contact with what matters
FedEx SenseAwareSM gives you real-time access to package information—from location to whether it’s been opened. Get proactive alerts if something happens.
Get protection for valuable packages
With FedEx Priority Alert®, you get a global service analyst who will take charge. They provide around-the-clock support and advanced shipment monitoring.
Next-level premium shipping support
Specialized, hazardous, and high-value shipments deserve more attention. Choose FedEx Custom Critical® services for truckload or less-than-truckload shipments.
Up-to-date alerts for weather delays
Receive daily email notifications about potential weather events. These emails help you plan around potential delays in FedEx pickups and deliveries the next day.
Temperature-controlled shipping and packaging
For some pharmaceuticals, even slight temperature fluctuations can be disastrous. Versatile and verifiable options address all your cold chain needs—in transit and in storage.* We can also provide a thermal blanket for freight shipments. Whether your shipment has to stay at room temperature or below freezing, we're here to preserve quality and accuracy.
Room temperature shipping (15° to 25° C)
Room temperature shipping
(15° to 25° C)
Extreme hot and cold temperatures can damage some items. Ship at a controlled room temperature so products arrive in top condition.
Cold chain shipping (2° to 8° C)
Cold chain shipping
(2° to 8° C)
Need to keep pharmaceuticals cold? See how our chilled boxes and specialized containers meet your quality and compliance needs.
Frozen shipping (-10° to -25° C)
Frozen shipping
(-10° to -25° C)
Frozen products require strict temperature control to remain viable. Learn more about how we create a safe frozen environment.
Deep frozen shipping (-150° to -195° C)
Deep frozen shipping
(-150° to -195° C)
Certain pharmaceuticals require extreme cold to remain viable. With our liquid nitrogen dry vapor technology, no dry ice is needed.
Ship with dry ice
Dry ice falls under the dangerous goods category. Learn how to prepare a shipment with dry ice. To ship it, contact our medical shipping experts.
Ship clinical samples with ease
Your samples must comply with certain laws governing packing and labeling. See how to properly ship liquid and dry materials.
Additional packaging options
Don’t need temperature control?
Find multiple packaging options for your healthcare delivery. Get complimentary packaging when you ship with FedEx expedited services.
Buy shipping supplies online
No matter what your shipping needs are, you’ll find FedEx packaging that fits. Check out a wide range of boxes, envelopes, and cushioning you can order online.
International healthcare shipping
Enter global medical markets with confidence and in compliance. Whether you need to ship ASAP or want more economical rates, we've got you covered. Fill out customs documents online, see if you need to file an Electronic Export Information (EEI) form, and more.
Meet international shipping regulations
Keeping up to date on import and export regulations can be time-consuming. Quickly get the latest regulatory and customs clearance info.
Resources for small businesses
Streamline shipping and grow your business
Streamline shipping and grow your business
From an updated shipping dashboard to marketing advice, find valuable resources and tools. We've made some changes so it's easier to browse, share, and succeed.
Automate your business' shipping with FedEx Ship Manager
Whether you're shipping just a couple of shipments per day, or hundreds, FedEx Ship Manager has options available to help you ship, track, and manage your shipments. See how it can help your business.
Support for every healthcare shipping journey
We know you have a lot to consider when you're choosing medical shipping
services. That’s why we have healthcare shipping experts who are here to help you
make these important decisions. We’ll work with you to create the best plan for
your unique needs.
For more information, contact your FedEx Sales Professional.
We know you have a lot to consider when you're choosing medical shipping services. That’s why we have healthcare shipping experts who are here to help you make these important decisions. We’ll work with you to create the best plan for your unique needs.
For more information, contact your FedEx Sales Professional.
* During delays, due to clearance or inclement weather, FedEx may route your cold shipments to a FedEx cold chain center. Contact your FedEx account representative to learn more.
* During delays, due to clearance or inclement weather, FedEx may route your cold shipments to a FedEx cold chain center. Contact your FedEx account representative to learn more.